When you pick up a generic pill from the pharmacy, you assume it works just like the brand-name version. But what happens to that pill over time? What if it breaks down before the expiration date? And how do we know it’s still safe to take? These aren’t just technical questions-they’re life-or-death concerns for millions of people relying on affordable medications every day.
What Stability Testing Really Means
Stability testing isn’t just about putting a bottle on a shelf and waiting. It’s a science that answers one core question: Does the medicine keep working the way it should until the day it’s labeled to expire? The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) require manufacturers to prove this through strict, documented studies. The goal isn’t just to meet a rule-it’s to make sure the drug doesn’t lose potency, change its physical form, or become harmful over time.There are four main areas tested: chemical, physical, microbiological, and functional stability. Chemical stability looks at whether the active ingredient breaks down into other substances. Even small amounts of these breakdown products-called impurities-can be dangerous if they build up. For example, ICH Q3B guidelines say unknown impurities must stay below 0.1% of the total drug content. That’s not a lot. One wrong step in manufacturing or packaging can push it over.
Physical stability checks things you can see or feel: color, texture, smell, whether tablets crumble, or if a liquid turns cloudy. For injectables, it’s about whether particles clump together. In nanoparticle drugs-like those used for cystic fibrosis-agglomeration above 200 nanometers makes the treatment useless. The whole point of making particles tiny is so they can reach the right cells. If they clump, they can’t.
Microbiological stability matters for anything that contains water. Preservatives can weaken over time, allowing mold or bacteria to grow. USP <61> and <62> set limits: non-sterile products can’t have more than 100 colony-forming units per gram. That sounds low, but it’s easy to exceed if humidity control fails during storage.
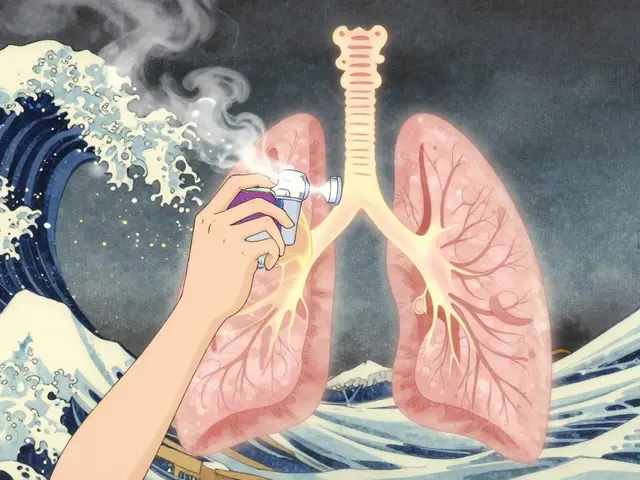
Functional stability applies to delivery systems like inhalers or patches. A metered-dose inhaler must deliver exactly 90-110% of the labeled dose every time. If the propellant leaks or the valve clogs, the patient gets less medicine-even if the pill still looks fine.
How Long Is “Long Term”? And Why Accelerated Testing Can Mislead
The standard test lasts 24 to 36 months under real-world conditions: 25°C and 60% humidity. That’s called long-term testing. But waiting three years to find out if a drug is stable isn’t practical. So companies use accelerated testing: 40°C and 75% humidity for six months. The idea is that heat speeds up degradation, so if the drug survives that, it should last longer.But here’s the problem: heat doesn’t always mimic real aging. A 2020 case from a pharmaceutical quality team showed an accelerated test found zero degradation. The product passed. But when stored at room temperature, it started crystallizing after 24 months-something the high-heat test never revealed. Why? Because crystallization happens through a slow polymorphic transition, not chemical breakdown. The accelerated test missed it entirely.
Dr. Kim Huynh-Ba, a stability expert with 25 years at the FDA, warns: “Performing accelerated testing at very high temperatures for a short time and expecting to extrapolate results to a long expiration date is risky. The degradation mechanism at 40°C may be completely different than at 25°C.”
That’s why regulators only allow extrapolation up to twice the length of the real-time data-and only if there’s no sign of change. Most generic drugs get approved based on 12 months of real-time data, meaning their 24- or 36-month shelf life is largely predicted, not proven.
Why Generic Drugs Are More Vulnerable
Generic drugs are supposed to be identical to brand-name versions. But they’re not. They use different fillers, binders, coatings, and manufacturing processes. These differences might seem minor, but they affect how the drug reacts to heat, light, and moisture.A 2020 FDA study found that 17.3% of generic levothyroxine products had stability issues not seen in Synthroid, the brand-name version. Why? Moisture. Some generics didn’t have adequate packaging to block humidity. Over time, the active ingredient degraded, leading to inconsistent dosing. Patients taking it for thyroid conditions could end up with symptoms like fatigue, weight gain, or heart palpitations-even though the pill looked fine.
Another example: generic insulin pens. Some use plastic components that absorb moisture differently than the original. That can cause the insulin to clump or lose potency faster. The FDA has issued multiple safety alerts on this.
Generic manufacturers often cut costs to compete on price. That means cheaper packaging, fewer stability tests, or skipping certain environmental stress tests. It’s not illegal-but it’s risky. And patients don’t know the difference.

Storage Isn’t Just “Room Temperature”
The label says “store at room temperature.” But what does that mean? The FDA defines it as 15-30°C (59-86°F). That’s a huge range. A garage in Arizona in summer hits 45°C. A bathroom in winter might drop to 10°C. Neither is ideal.Quality professionals say the most common reason for FDA warning letters is poor storage documentation. Just writing “room temperature” on a report gets you cited every time. You need to log exact temperatures, humidity levels, and how long the product was exposed to each condition.
And it’s not just about storage. Distribution matters too. In low-income countries, the WHO found that 28.7% of medicines fail stability testing-not because they were poorly made, but because they sat in unrefrigerated trucks for weeks in 35°C heat. In high-income countries, that number is under 1.5%.
Even in Australia, where temperatures are rising, warehouses that used to stay cool now hit 30°C for over 80 days a year. A 2022 MIT study predicts that by 2050, average drug shelf life could drop by nearly 5 months just because of climate change.
What Happens When Stability Fails?
The consequences aren’t theoretical. In 2022, a major recall of a generic antibiotic happened because preservatives degraded, allowing bacterial growth in the liquid suspension. Parents gave it to their kids-and some got sick.The Parenteral Drug Association found that 62.7% of stability professionals experienced a product recall in the last five years. The top cause? Microbial growth due to changes in water activity. That’s not a manufacturing flaw. It’s a stability failure.
For food products, the end of shelf life is when consumers say it tastes bad or looks off. For drugs, it’s when the active ingredient drops below 90% of its labeled potency. That’s the legal limit. But if a patient needs 100% to control their condition, 90% might not be enough.
Take blood pressure medication. If the active ingredient drops to 85%, the patient’s BP might spike without warning. No symptoms. No alert. Just a silent failure.

What’s Changing-and What’s Still Broken
New tools are emerging. Risk-Based Predictive Stability (RBPS) models, tested by Amgen and Merck, cut shelf-life determination time by 30%. They use data from multiple stress conditions to predict how a drug will behave over years. But regulators still don’t fully accept them. The ICH Q12 guideline, effective since late 2023, allows post-approval changes with less testing-but only if companies can prove safety. Few have the resources to do it.The FDA’s pilot program for continuous manufacturing showed shelf life could be determined 40% faster. That’s promising. But it only applies to a small number of new drugs.
Meanwhile, the cost of proper stability testing is high. The average investment per product is $1.2 million. That’s why small generic manufacturers cut corners. And patients pay the price.
What You Can Do
You can’t test your own pills. But you can protect yourself:- Check the expiration date. Don’t use pills past it-even if they look fine.
- Store medications in a cool, dry place. Not the bathroom. Not the car. A closet away from heat and humidity is best.
- If your generic drug seems less effective, talk to your pharmacist. Ask if there’s a different manufacturer. Some brands are more reliable.
- Report side effects. If you notice changes in how a medication works, report it to your doctor and to the FDA’s MedWatch program.
Stability isn’t magic. It’s science. And science takes time, money, and attention. The system isn’t perfect. But awareness is the first step toward safer medicine.
How is shelf life determined for generic drugs?
Shelf life for generic drugs is determined through stability testing that follows ICH Q1A(R2) guidelines. Manufacturers test three batches under real-world conditions (25°C, 60% RH) for up to 36 months. Accelerated testing (40°C, 75% RH) for six months is used to predict long-term behavior, but results must be validated with real-time data. Regulatory agencies require proof that the drug maintains potency, purity, and physical integrity throughout its labeled shelf life.
Can expired generic drugs be dangerous?
Yes. While many expired drugs lose potency rather than become toxic, some can degrade into harmful compounds. For example, tetracycline antibiotics can break down into nephrotoxic substances. Insulin and nitroglycerin lose effectiveness rapidly after expiration, risking serious health consequences. Even if a pill looks unchanged, chemical degradation may have occurred unseen.
Why do some generic drugs fail stability tests while brand-name ones don’t?
Generics often use different inactive ingredients-like fillers, coatings, or preservatives-that affect how the drug responds to heat, moisture, and light. Packaging quality also varies. A 2020 FDA study found 17.3% of generic levothyroxine products had stability issues due to inadequate moisture protection, while the brand-name version did not. Manufacturing processes differ too, leading to variations in crystal structure or particle size that impact long-term stability.
Is accelerated stability testing reliable?
Accelerated testing can be useful but is not always reliable. It works well for chemical degradation, but fails to predict physical changes like crystallization, polymorphic transitions, or particle agglomeration. In one case, a generic drug passed accelerated testing but crystallized after 24 months at room temperature. Regulators allow extrapolation only if real-time data shows minimal change. Overreliance on accelerated data without validation can lead to false safety claims.
What should I do if I suspect my generic medication isn’t working?
If you notice a change in how your medication works-like increased symptoms, side effects, or lack of effect-talk to your doctor. Ask if your prescription was switched to a different generic manufacturer. Some brands have better stability records. Keep your medication stored properly, check the expiration date, and report concerns to your pharmacist or through the FDA’s MedWatch program. Don’t assume all generics are the same.




Mark Kahn
November 21, 2025 AT 13:03Hey everyone, just wanted to say this post is a wake-up call. I’ve been taking generic blood pressure meds for years and never thought twice about storage-until my aunt had a scary spike last year. Now I keep mine in a sealed container in the closet. Small change, big difference.
Also, if you’re on thyroid meds, DO NOT switch generics without talking to your doc. I learned that the hard way.
Leo Tamisch
November 22, 2025 AT 08:12How quaint. We’ve reduced life-saving pharmacology to a grocery aisle comparison between ‘brand’ and ‘store brand.’ The irony? We demand iPhone-level precision from our medications while treating them like discount toilet paper. 🤡
Perhaps if we stopped outsourcing our health to corporate arbitrage, we wouldn’t need to worry about 0.1% impurities. But then again-capitalism.
Daisy L
November 23, 2025 AT 00:49THIS. IS. NONSENSE. 😡 We’re letting Chinese and Indian factories cut corners on our LIFE-SAVING DRUGS?! And then we wonder why people get sick?! The FDA’s been asleep at the wheel for DECADES! They approve generics based on 12 months of data?! That’s not science-that’s a gamble with human lives!
My cousin died because her generic insulin degraded in her car during a heatwave. And now you want me to ‘just store it in a closet’?! NO. WE NEED REGULATIONS. NOW.
Eliza Oakes
November 24, 2025 AT 03:14Oh please. You’re all acting like generic drugs are some new invention. They’ve been around since the 70s. The fact that you’re shocked by degradation means you’ve never read the label. ‘Store in a cool, dry place’ isn’t a suggestion-it’s a warning. And if you’re keeping meds in your bathroom? You’re the problem.
Also, ‘brand-name’ drugs? They’re just the original generics that got lucky with marketing. Same chemistry. Different price tag.
Shawn Sakura
November 25, 2025 AT 13:36Thank you for writing this. I’m a nurse, and I see patients every day who don’t know the difference between ‘expired’ and ‘still good.’ I’ve had people bring me pills that were 5 years past their date-because ‘it still looks fine.’
It’s not just about potency. It’s about trust. When your body relies on a pill to keep you alive, you deserve to know it won’t let you down.
Also-please, PLEASE store meds away from the sink. Humidity is the silent killer.
Swati Jain
November 26, 2025 AT 19:20Let’s cut through the jargon: generic drugs are the MVPs of global healthcare. Without them, 80% of the world couldn’t afford treatment. Yes, packaging and fillers vary. Yes, some batches fail. But the system works-most of the time.
Instead of fearmongering, let’s demand transparency: QR codes on bottles linking to stability data. Real-time temp logs from distributors. That’s the future. Not paranoia.
And for the love of science-stop blaming India. They make 40% of the world’s generics. If they fail, the whole system collapses. They’re not villains-they’re the backbone.
Florian Moser
November 28, 2025 AT 03:02One of the most important public health pieces I’ve read this year. The fact that shelf life is extrapolated from 12 months of data is deeply concerning. Regulatory agencies are operating on assumptions, not evidence.
And climate change is an unaddressed variable. By 2050, we may be looking at 5-month shelf life reductions. That’s not theoretical-it’s a logistical crisis waiting to happen.
Thank you for the actionable advice. I’ve already moved my meds to the bedroom closet.
jim cerqua
November 29, 2025 AT 04:34MY BROTHER DIED BECAUSE OF A GENERIC DRUG.
He was on a generic version of warfarin. Took it for 3 years. Looked fine. Expiration date? Still good. But the coating degraded. The pill didn’t dissolve right. Blood clots. Stroke. Gone in 48 hours.
The FDA knew. The manufacturer knew. But they didn’t tell anyone. Because profit > people.
Don’t trust the label. Don’t trust the pharmacy. Trust nothing.
And if you’re still using generics? You’re playing Russian roulette with your life.
Cooper Long
November 29, 2025 AT 19:42Stability testing is not an exact science. It is a probabilistic model based on controlled environments. Real-world conditions are chaotic. Temperature fluctuations, humidity spikes, transport delays-all these factors are not accounted for in regulatory filings.
The solution is not to abandon generics. It is to implement real-time monitoring: smart packaging with temperature sensors, blockchain-tracked logistics, and open-access stability data.
We have the tools. We lack the will.
Logan Romine
December 1, 2025 AT 02:35So let me get this straight: we’re okay with a 10% drop in potency because ‘it’s still legal’? 😂
Imagine if your car’s brakes lost 10% effectiveness. Would you still drive it? Would you say, ‘eh, it’s still under warranty’?
Drugs aren’t widgets. They’re life support. And we treat them like expired yogurt.
Also, ‘room temperature’ is a joke. My apartment hits 32°C in summer. Thanks for the safety tip, FDA.
Franck Emma
December 2, 2025 AT 06:45They’re lying to us.
That’s it.
I’m done.
Noah Fitzsimmons
December 2, 2025 AT 18:07Oh wow, you actually read the ICH Q3B guidelines? How adorable. You think you’re the first person to notice that generics have different excipients? Congrats. You discovered the pharmaceutical industry is a business.
But here’s the real question: why do you think brand-name drugs don’t degrade too? They do. They just have better packaging and a PR team that says ‘trust us.’
Also, your ‘cool closet’? That’s not even a real temperature. You need a data logger. Or are you just here for the drama?