Ever picked up a prescription and wondered why the pill has three different names? One looks like a chemistry equation, another is short and familiar, and the third sounds like a superhero alias? That’s drug nomenclature in action - a quiet but vital system designed to keep you safe. It’s not just about labels. It’s about preventing mistakes that could land you in the hospital.
Why Drug Names Even Exist
Imagine walking into a pharmacy in Sydney, Tokyo, or Toronto and asking for the same medicine. If every country used its own naming system, you’d get different labels, different spellings, maybe even different drugs. That’s not hypothetical - it happened before the 1950s. Medication errors were common, and confusion between similarly named drugs caused serious harm.
In 1953, the World Health Organization stepped in and launched the International Nonproprietary Names (INN) Programme. Their goal? One name, one drug, worldwide. Today, over 10,000 drugs have standardized names. Each year, about 200 new ones get added. The system works because it’s built on logic, not luck.
The Three Layers of a Drug’s Identity
Every drug has three names - each serving a different purpose. Think of them like a person’s full legal name, their nickname, and their stage name.
- Chemical name: The full scientific blueprint
- Generic name: The official, universal identifier
- Brand name: The commercial label you see on the box
They’re not interchangeable. Each has rules. Break the rules, and you risk confusion - and worse.
Chemical Names: The Molecular Blueprint
This is the most precise name a drug can have. It’s built using rules from the International Union of Pure and Applied Chemistry (IUPAC). It tells you exactly how the atoms are arranged - like a map of the molecule.
Take propranolol. Its chemical name is 1-(isopropylamino)-3-(1-naphthyloxy) propan-2-ol. That’s 48 characters. Try saying that in a hurry during an emergency. Good luck.
Chemical names are used in labs, patents, and scientific papers. No doctor writes this on a prescription. No pharmacist dispenses based on this. It’s too long, too technical, and too slow. But it’s the foundation. Everything else is built on this structure.
Generic Names: The Global Standard
This is the name you’ll see on the bottle when you buy the cheapest version of the drug. It’s also the name doctors write in charts and pharmacists use to fill prescriptions.
Generic names aren’t random. They follow strict patterns. The ending - called a stem - tells you the drug’s class. The beginning - the prefix - makes it unique.
Here’s how it works:
- Drugs ending in -prazole are proton pump inhibitors: omeprazole, lansoprazole, pantoprazole.
- Drugs ending in -tinib are tyrosine kinase inhibitors: imatinib, sunitinib, dasatinib.
- Drugs ending in -mab are monoclonal antibodies: adalimumab, rituximab, trastuzumab.
- Drugs ending in -citinib are Janus kinase inhibitors: tofacitinib, upadacitinib.
These stems are like a secret code. If you know them, you can guess what a drug does - even if you’ve never heard of it before.
The USAN Council (United States Adopted Names) and WHO’s INN committee work together to approve these names. They reject about 30% of proposed names. Why? Because even small similarities can cause errors.
For example, the name glipizide was almost rejected because it sounded too much like glimepiride. Both treat diabetes. Mix them up, and you could get dangerously low blood sugar. That’s why the council spends months testing names for pronunciation, spelling, and sound - especially in languages like Spanish, Mandarin, and Arabic.
Dr. Robert M. Goggin, former head of the USAN Council, found that properly designed generic names reduce medication errors by 27%. That’s not a small number. It’s life-saving.
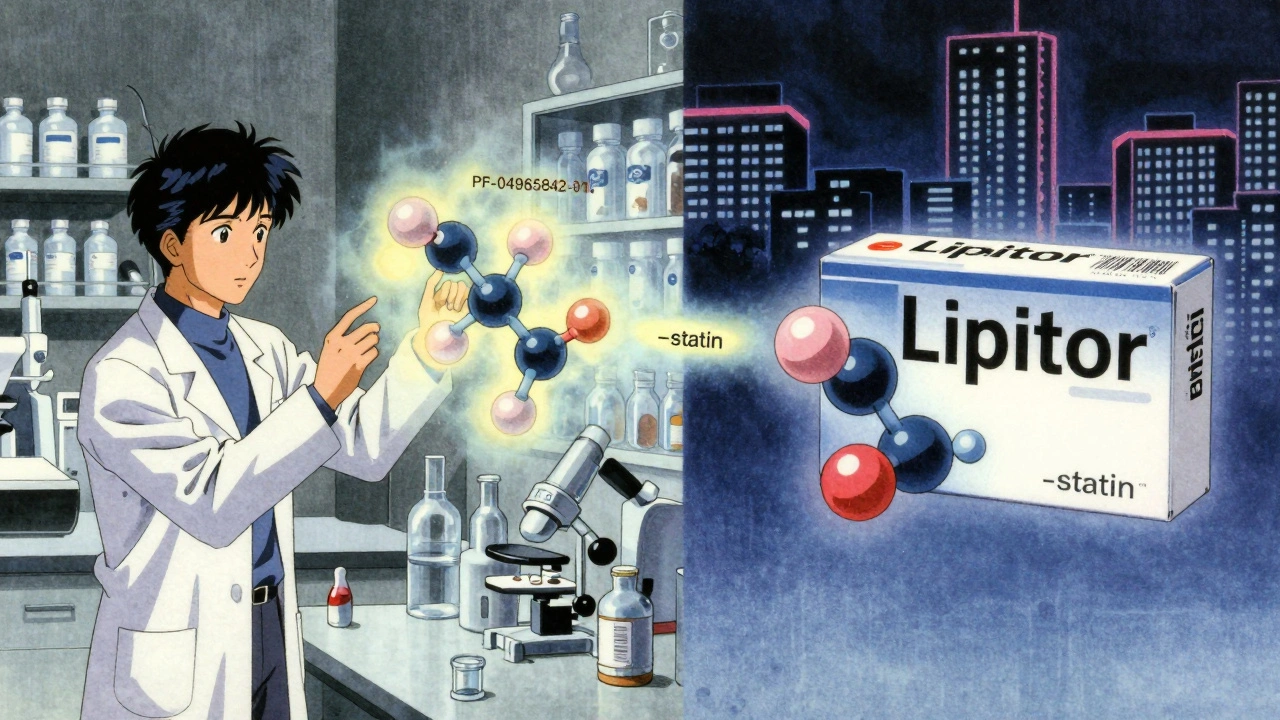
Brand Names: The Marketing Mask
This is what you see on TV ads. The catchy, memorable name. Like Lipitor, Nexium, or Xarelto.
Pharmaceutical companies spend millions developing these. They test hundreds of names. Only one makes it through the FDA’s review.
The FDA’s rules are strict:
- No name can sound like another drug (even if it’s for a different condition).
- No name can suggest the drug cures everything (like “CureAll” or “InstantRelief”).
- The generic name must appear next to the brand name in all ads.
One in three proposed brand names gets rejected. Why? Because of confusion risks. In 2022, the FDA reported 347 medication errors linked to similar-looking or sounding brand names. One case involved Hydrea (hydroxyurea) and Hycamtin (topotecan) - both used for cancer. A nurse gave the wrong one. The patient didn’t survive.
Brand names also have to be trademarkable. That’s why they often sound like sci-fi words. They can’t be generic terms. You can’t call a blood thinner “Aspirin” if that’s already a brand. So companies invent names like Plavix or Pradaxa.
But here’s the catch: brand names change when the patent expires. The same drug becomes generic. The active ingredient stays the same. The pill might look different - color, shape, size - because the brand company owns the design. But the medicine inside? Identical.
Company Codes: The Hidden Name
Before a drug gets a generic or brand name, it has a code. Pfizer uses “PF” followed by numbers - like PF-04965842-01. That’s abrocitinib before it had a name. AbbVie uses “ABBV,” Merck uses “MK.”
These codes are internal. Used in research papers, clinical trials, and patent filings. They’re not meant for patients. But they’re critical for tracking. If a side effect shows up in a trial, scientists trace it back to the exact compound using that code.
These codes follow a pattern: the first part is the company’s prefix, the middle digits identify the molecule, and the last two digits often indicate the salt form or version. It’s like a serial number for your medicine.
Why This Matters to You
You might think, “I don’t need to know all this.” But you do.
When you get a new prescription, ask: Is this the brand or the generic? If your doctor says “Lipitor,” you might pay $200 a month. If they say “atorvastatin,” you could pay $10. Same drug. Same effect.
But here’s the problem: 68% of patients say generic names are confusing. They don’t know what the suffix means. They see “metformin” and think it’s something totally different from “glucophage” - even though they’re the same thing.
Pharmacists know the system. They see the stem and know the class. They can warn you if a new prescription might interact with something you’re already taking. But you need to understand enough to ask the right questions.
Also, if you’re traveling overseas, you’ll see different brand names. In Australia, you might get “Lipitor.” In India, it’s “Atorva.” Same pill. Different label. Knowing the generic name keeps you safe.

What’s Changing Now
Drug science is moving fast. New types of medicines don’t fit old naming rules.
RNA-based therapies? New stem: -siran. Peptide-drug conjugates? New stem: -dutide. Protein degraders? Proposed stem: -tecan.
The WHO and USAN Council are updating their rules every year. They’re using AI now to scan 15,000 existing drug names in seconds to spot potential mix-ups. In 2022, this system cut naming errors by 42%.
By 2030, nearly 5% of new drugs will be protein degraders - a huge jump from 1.2% today. That means more new stems. More names to learn. But also, better safety.
What You Can Do
You don’t need to memorize all the stems. But here’s what you can do right now:
- Always ask your doctor or pharmacist: What’s the generic name?
- When you refill a prescription, check the label. Does it say the generic name? If not, ask why.
- If you’re on multiple drugs, write down the generic names. Keep them in your phone or wallet.
- Don’t assume brand = better. Generic drugs are required by law to work the same way.
- Use apps or websites that show you the generic equivalent of a brand name. GoodRx and Drugs.com do this well.
Medication errors are still a problem. But standardized naming has cut them by nearly 20% since 2010. That’s thanks to this system. You’re part of it - by asking questions, by paying attention, by choosing generic when you can.
Common Confusions and How to Avoid Them
Here are real examples of mix-ups that happened - and how to prevent them:
- Hydroxyzine vs. Hydralazine: One treats anxiety. The other treats high blood pressure. Sounds similar? They’re not the same. Always spell it out.
- Fluoxetine (Prozac) vs. Floxacillin (an antibiotic): Different endings, but same first syllable. If you’re allergic to penicillin, this mix-up could be dangerous.
- Metoprolol vs. Metformin: One slows your heart. The other lowers blood sugar. Both start with “meta.” Don’t assume.
Always double-check. If a name looks or sounds like another drug you’re taking - speak up. Your pharmacist is trained to catch these. But they can’t read your mind.
Why do drugs have three different names?
Drugs have three names because each serves a different purpose. The chemical name describes the exact molecular structure - used by scientists. The generic name is the standardized, worldwide identifier used by doctors and pharmacists for safety and clarity. The brand name is chosen by the company for marketing and is protected by trademark. The system prevents confusion and ensures consistency across countries and languages.
Are generic drugs as effective as brand-name drugs?
Yes. By law, generic drugs must contain the same active ingredient, strength, dosage form, and route of administration as the brand-name version. They’re tested to prove they work the same way in your body. The only differences are in inactive ingredients - like color, shape, or filler - which don’t affect how the drug works. Generic drugs save money without sacrificing safety or effectiveness.
How are generic drug names chosen?
Generic names are created by international bodies like the WHO’s INN Programme and the USAN Council. They use standardized stems - endings that indicate the drug’s class - like “-prazole” for acid reducers or “-tinib” for cancer drugs. The prefix makes each drug unique. Names are tested for pronunciation, spelling, and similarity to existing drugs. About 30% of proposed names are rejected to prevent dangerous mix-ups.
Why do brand names sound so strange?
Brand names are designed to be memorable, trademarkable, and legally distinct. They can’t sound too much like other drugs or suggest unrealistic benefits. Companies test hundreds of names before submitting one to the FDA. Many are rejected because they’re too similar to existing names. That’s why you see unusual words like “Xarelto” or “Nexium” - they’re built to pass strict safety checks.
Can drug names change after they’re approved?
The generic name rarely changes once approved. But brand names can be retired when patents expire and generics enter the market. Sometimes, a company will rebrand a drug for a new use - like switching from “Viagra” (for erectile dysfunction) to “Revatio” (for pulmonary hypertension), even though the active ingredient is the same. The generic name stays the same: sildenafil.
How do I know if I’m getting the generic version?
Check the label on your prescription bottle. It will list the generic name first, followed by the brand name in parentheses if it’s a brand. If it says “atorvastatin” and not “Lipitor,” you’re getting the generic. You can also ask your pharmacist - they’re required to tell you if you’re getting a generic substitute. In most cases, generics are automatically substituted unless the doctor writes “dispense as written.”
Final Thought: Knowledge Is Your Shield
Drug nomenclature isn’t just for scientists or pharmacists. It’s a safety net. The more you understand how names work, the less likely you are to be caught in a mistake. You don’t need to be an expert. Just be curious. Ask questions. Read the label. Know the generic name. That’s how you take control - not just of your medicine, but of your health.







Jennifer Patrician
December 4, 2025 AT 07:24Of course the WHO and FDA are 'protecting' us. Meanwhile, Big Pharma is secretly coding all their drugs with hidden tracking chips under the guise of 'standardized naming.' You think '-mab' is just a stem? Nah. It's 'Monitor And Broadcast.' They're watching your vitals through your prescriptions. I've seen the leaked documents. The same people who made you believe aspirin is safe also invented the 5G myth. Wake up.
Mark Curry
December 4, 2025 AT 16:25It's kind of beautiful, really. A system built not for profit, but for safety. Like a universal language for life-saving things. I never thought about how much work goes into picking a name that won't kill someone. It's quiet, unglamorous science - but it saves lives every day. :)
Reminds me of how we name stars. Not for fame. For order.
Manish Shankar
December 6, 2025 AT 06:16Respected colleagues, I find this exposition on pharmaceutical nomenclature to be profoundly enlightening. In my practice in Mumbai, I have observed that patients frequently confuse brand and generic names due to linguistic and educational disparities. The adoption of standardized stems, particularly in multilingual environments, significantly reduces dispensing errors. I commend the WHO and USAN Council for their rigorous, evidence-based approach. This is public health at its most elegant.
May I suggest that future educational materials be translated into regional Indian languages to further enhance comprehension?
Lynette Myles
December 6, 2025 AT 11:45They reject 30% of names. But they never reject the ones that sound like 'CureAll' because they're already selling them as supplements. You're being lied to.
Annie Grajewski
December 7, 2025 AT 01:40so like… the whole ‘-tinib’ thing? yeah cool. but why does it sound like a pokemon? like ‘sunitinib’?? who named this? a 12-year-old on acid??
and don’t even get me started on ‘Xarelto’ - that’s not a drug, that’s a spaceship from a bad sci-fi movie. i swear, if i hear ‘Pradaxa’ one more time i’m gonna scream.
also, generic = cheaper = better? yeah right. my cousin took ‘atorvastatin’ and it made her hallucinate cats. brand name didn’t. coincidence? i think not.
Katie Allan
December 8, 2025 AT 12:43This is one of the most important things most people never learn - and it could literally save your life. I’ve taught my elderly parents to always ask for the generic name and write it down. It’s not about being cheap. It’s about being informed.
If you’re on multiple meds, keep a list. Use a note app. Share it with your pharmacist. You don’t need a medical degree to be your own advocate. Just curiosity and a little courage.
Thank you for writing this. So many people need to see it.
Kylee Gregory
December 10, 2025 AT 01:39I’ve always wondered why some drugs have such weird names, and this breaks it down so clearly. It’s fascinating how much thought goes into something most people ignore.
It makes me think - if we can create a system this precise for medicine, why can’t we apply similar logic to other areas? Like politics? Or internet usernames?
Maybe we’re just too used to chaos.
Lucy Kavanagh
December 10, 2025 AT 06:14Let me guess - the WHO is just a front for the UN’s global drug control agenda. They’re not trying to standardize names… they’re trying to standardize *you*. Why else would they push this in every country? Even India? Even the UK?
And don’t tell me it’s for safety. They banned iodized salt too. Then they made you pay for it. Same game.
Next they’ll make you wear a wristband with your generic drug name. You think I’m joking?
Chris Brown
December 11, 2025 AT 23:27It is deeply troubling that the public is encouraged to accept generic pharmaceuticals as equivalent to branded products without understanding the subtle, yet potentially consequential, differences in bioavailability and excipient composition. The FDA’s approval process, while ostensibly rigorous, is compromised by corporate lobbying and regulatory capture. To suggest that cost savings justify this compromise is not merely irresponsible - it is morally indefensible.
One must ask: who benefits from the erosion of brand integrity? Not the patient. Not the physician. Only the oligarchs who control the supply chain.
Dispense as written - or suffer the consequences.